

LymphActivist's Site
Dedicated to Lymphedema Patients and the Therapists Who Treat Them


LymphActivist's Site
Dedicated to Lymphedema Patients and the Therapists Who Treat Them
Does Incorrect Coding Lead to Medicare Denials?
By Robert "Bob" Weiss, M.S.
As the Centers for Medicare & Medicaid Services (CMS) attempts to streamline the Medicare claims reimbursement system to achieve cost efficiencies through a greater dependence on computers, is the basic function of Medicare being subverted to the goals of administrative efficiency? Decisions are sometimes made without proper anticipation of unintentional consequences and medical decisions are usurped by administrative staff using coded rules. I would like to focus on how this evolving process has grown over the years and how it affects lymphedema patients today.
Administering Medicare
Medicare was established and operates under "Title XVIII" of the Social Security Act (the Act). Congress has empowered CMS (formerly the Health Care Financing Administration, HCFA) to administer Medicare through contracts with health care and insurance contractors. CMS interprets the regulations in title XVIII and in the Code of Federal Regulations (CFR) and transforms them into national policy, documented in an extensive online publication system. Regional Medicare administrative contractors develop local coverage determinations (LCDs) to apply the national rules at the local level.
What Determines Whether a Medicare Service is Covered?
Benefit Categories Relevant to Lymphedema Treatment
Each section of the Act defines, in terms of function, the criteria for a service or an item to fit into a covered benefit category. This is a concept that is little understood by CMS, leading sometimes to the denial of an item based on its form although it meets all the coverage criteria based on its function.
The specific sections of the Act that have primary impact on lymphedema beneficiaries are:1861(s)(5) Surgical Dressings, Splints and Casts: "surgical dressings, and splints, casts, and other devices used for reduction of fractures and dislocations";
1861(s)(6) and 1861(n) Durable Medical Equipment (DME): equipment which: can withstand repeated use (and be rentable); is primarily and customarily used to serve a medical purpose; is necessary and reasonable for the treatment of the patient's illness or injury or to improve the functioning of his or her malformed body member; generally is not useful to a person in the absence of an illness or injury; and is appropriate for use in the home and is used in the home.
1861(s)(8) Prosthetic Devices: "...replace all or part of an internal body organ...". The brief definition in the Act was expanded by CMS to: "... items which replace all or part of an internal body organ or replace all or part of the function of a permanently inoperative or malfunctioning internal body organ."
1861(s)(9) Orthotics and Prosthetics: "leg, arm, back, and neck braces [orthotics], and artificial legs, arms, and eyes [prosthetics]".
Note that "prosthetics" replace missing body parts, while "prosthetic devices" replace internal organs or their function.
Table 1
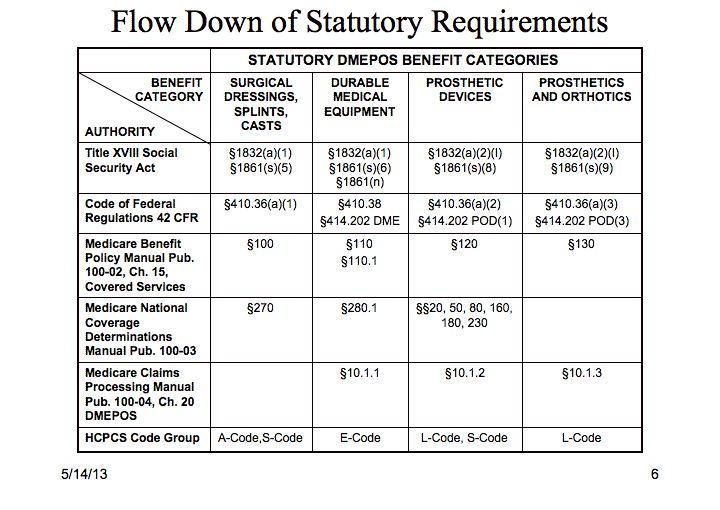
Coverage is conferred by §1832 based on whether an item falls within a benefit category defined in §1861. Note in Table 1 that "surgical dressings, splints and casts", "durable medical equipment", "prosthetic devices", and "prosthetics and orthotics" benefits have different defining functions, and give rise to different defining regulations in the Code of Federal Regulations and in Medicare policy manuals.
CMS Online Manual System (Lines 3, 4 & 5)The CMS Online Manual System, available at https://www.cms.gov/manuals/iom/list.asp, is used to administer CMS programs. The three manuals of interest in this discussion are Publications 100-02 Medicare Benefit Policy Manual, 100-03 Medicare National Coverage Determinations (NCD) Manual and 100-04 Medicare Claims Processing Manual. Relevant sections may be found in Table 1 for the four selected benefit categories.
Healthcare Common Procedure Coding System (HCPCS)
What is the HCPCS?"The HCPCS Level II coding system is a comprehensive, standardized system that classifies similar products that are medical in nature into categories for the purpose of efficient claims processing. Products are classified based on similarities in function and whether the products exhibit significant therapeutic distinctions from other products." [Ref. CMS.gov]. "Inclusion or exclusion of a procedure, product, or supply does not imply any coverage or reimbursement policy." [HCPCS Manual (July 2003)]
HCPCS Codes for Lymphedema Materials (Line 6)HCPCS codes are grouped by benefit category. In addition to the codes for supplies, DME and prosthetics/orthotics (A-codes, E-codes and L-codes respectively) there are special codes (S-codes) that are not covered by Medicare but are used by insurers to assist in claims processing. The code groups used by Medicare and industry for lymphedema items are shown on Table 2.
In 2006 CMS made an "administrative" change that radically altered the reimbursement situation for lower-limb lymphedema patients. At a meeting of the HCPCS Workgroup on May 24, 2005 the benefit category of graduated compression stockings was changed from "prosthetic procedures" to "surgical dressings". The total justification in the record was "These items are not orthotics, but some of the items are covered under the surgical dressing benefit."
In 2007 this writer made a formal request (HCPCS Change Request #07.109) to restore the lower limb garment codes to the L-Code group and to move the upper limb garments and compression bandaging supplies from the S-code to the L-code group based on their medical function rather than their form. The request was rejected on the basis that "No insurer (i.e. Medicare, Medicaid, Private Insurance Sector) identified a national program operating need to establish unique codes to distinguish all the products listed in this application. Existing codes adequately describe the array of products available." A request by MEDI USA to restore the L-codes for graduated compression stockings (HCPCS Change Request #11.124) was rejected November 2011.
Denial of Lymphedema Garment Claims
Medicare Contractor-Cited Bases For DenialsPrior to 2006 denials were rendered most commonly on the basis that support hose did not meet the rentability requirement for DME, as stated in §1861(n) of the Act and §280.1 of the NCD, or that fabric supports were not covered as splints and braces. Since 2006 Durable Medical Equipment Medicare Administrative Contractors (DME MACs) commonly reject lymphedema compression garments because they "do not fall into a defined benefit category" or because they do not meet the coverage requirements in the NCD for durable medical equipment, or in the LCD for surgical dressings or breast prostheses.
In the absence of LCDs covering prosthetic devices benefits, and the lack of appropriate HCPCS L-codes, lymphedema garments are billed with inappropriate billing codes that guarantee that they will be denied.
The four DME MACs have not assumed their contractual responsibilities to make coverage determinations for prosthetics/orthotics/prosthetic devices within their jurisdictions in the absence of national or local coverage determinations (CMS Pub. 100-03, Chap. 1, Part 1, §A and 2009 Jurisdiction List for DMEPOS HCPCS Codes).
Plan of ActionThere are at least two approaches to solving the lymphedema compression bandage, garment and device coverage problem.
Achieve change within current statutes — Prepare a formal request for CMS to create a new National Coverage Determination on the Treatment of Lymphedema, redefining and expanding the proposed National Policy submitted to HCFA in 2000 by Robert Weiss. The resulting NCD would establish and coordinate coverage for: lymphedema compression bandages, garments and devices as prosthetic devices under current Medicare law; lymphedema therapy including therapist payment for bandaging, garment measurement and fitting, and patient education; medically-appropriate sequential pneumatic compression devices. HCPCS Codes must be changed to recognize the unique function of items and materials used in the treatment of lymphedema.
Change Title XVIII — Achieve the same goals with a federal law which would create a new benefit category for the medical materials required in the compression therapy of lymphedema, thereby leaving in place the LCDs and policies for the other benefits. I started this alternate path in 2001 after my proposed lymphedema treatment NCD and proposed changes to the DME Supplier Manual were rejected by HCFA. The Lymphedema Diagnosis and Treatment Cost-Saving Bill, which I wrote in 2002, and which created a new benefit category, was eventually introduced in Congress in 2010. Unfortunately the 2010 bill was stripped of some of its desirable provisions when it was re-introduced in 2011.
Caveat
The preceding material is the opinion of a volunteer patient advocate and does not reflect the positions of any organization or governmental agency. The statements made herein are based on personal experience and research, the writer's laic interpretation of relevant statutes, and determinations of selected Administrative Law Judges. The foregoing material is not offered and should not be taken as medical or legal advice.